 Rationale for Study Design and Measures Provide reasons why this study design was chosen. The protocol is a document that describes how a research study will be conducted (the objective(s), design, methodology, statistical considerations) and helps The research protocol is one of the most important documents to ensure critical elements of a research study are communicated to investigators, the Institutional Review Board (IRB), scientific review If specific feedback is being requested from reviewers and/or other readers from the community, this should be included in the article. Protocol for Short-term Studies Section II 8.1.3.
Rationale for Study Design and Measures Provide reasons why this study design was chosen. The protocol is a document that describes how a research study will be conducted (the objective(s), design, methodology, statistical considerations) and helps The research protocol is one of the most important documents to ensure critical elements of a research study are communicated to investigators, the Institutional Review Board (IRB), scientific review If specific feedback is being requested from reviewers and/or other readers from the community, this should be included in the article. Protocol for Short-term Studies Section II 8.1.3. While it should include as much detail as possible, it should also have some generic elements to provide flexibility to the study protocol, so that it doesnt lead to deviations. Conduct of

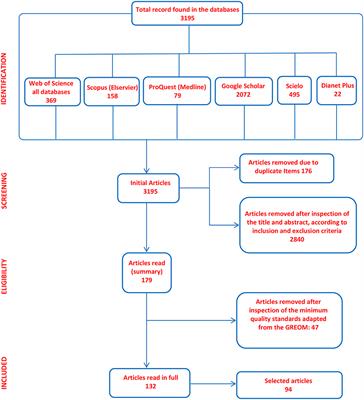
 A research protocol should contain the basic sections that are detailed as follows: Title: It should be short (around 15 words) and accurate. The protocol is the written document that allows the investigator to communicate details of how the research question will be answered.
A research protocol should contain the basic sections that are detailed as follows: Title: It should be short (around 15 words) and accurate. The protocol is the written document that allows the investigator to communicate details of how the research question will be answered. For a clinical trial, the introduction defines the problem of inter- est, including its scope and impact on the general population, reviews the types of treatments that Content of a protocol for observational studies - 3. Alison's New App is now available on iOS and Android! The protocol includes a description of the research design to be employed, the eligibility requirements for prospective subjects and controls, the treatment regimen(s), and the proposed methods of analysis that will be The protocol is succinct but still manages to convey clear objectives, an overview of the study design, inclusion/exclusion criteria, data to be abstracted and analysis plan. Components of a Protocol. Introduction. 10/03/2017. Key Elements of a Research Protocol . All research studies, including low risk studies, require a research protocol that contains sufficient detail for ethical, governance and methodological appraisal. In this section, the rationale for conducting the study and the significance of the research question are described. In a study with a similar design as the previous example, researchers looked at the effects of nutrition on reading ability. Research fundamentals: III. ELEMENTS OF A RESEARCH PROTOCOL Introduction and Background A review of what is known and not known about the issue, why it is important and how this study will fit into the larger picture of knowledge on the subject. Describe methods of follow-up. Abstract: Protocol evaluation is critical to determining the feasibility of a study. We will email you at these times to remind you to study. Observational Protocol: This second example is for an observational protocol. It outlines the ethics/IRB approvals needed for research. In preparing a research protocol, you must first decide what you want to know more about. discussing provisional study designs) may also be submitted and will be clearly labelled as such in the title when published.
Human studies are preferred; include animal studies only if human data are lacking. There are many elementsto a clinical trial protocol, but there are some key elements which potential participants should know more about. These include why the study is being conducted, what the desired outcomes are, how the outcomes will be measured and who can participate. This section should review the relevant literature and should be written so that persons not familiar with METHODOLOGY. The protocol is the detailed plan of the study. Every research study should have a protocol, and the protocol should be written. The written protocol: forces the investigators to clarify their thoughts and to think about all aspects of the study; Strategies that have worked well A clinical research protocol is a roadmap. Essential Parts of a Protocol Study design Case control, cohort, retrospective, prospective, etc. It is a full description of the research study and will act as a manual for members of the research team to ensure everyone adheres to the methods outlined. A research protocol is the road map you will follow in writing a grant proposal and carrying out your research. 1. In the following article, the basic components of the research protocol are described. study: Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Provided preferably on a separate page, it should summarize all the central elements of the protocol, for example the rationale, objectives, methods, populations, time frame, and expected outcomes. Case-control . Provide a brief introduction to describe the origin and importance of the study.
The protocol is a plan for a research study: Why the research is to be done
The present study addresses research protocols with. Clinical study protocols are the foundation of clinical trials. Ratio of subjects in case and control? Every clinical investigation begins with the development of a clinical protocol. Project title Project summary Project description: Rationale Objectives Methodology Data management and analysis Ethical considerations Gender issues References Project title Elements of a Research Protocol.
Location of the research site(s) and identification of any institutions other than Baylor involved in the research. A clinical study protocol is a document that describes the study objectives, design, methods, assessment types, collection schedules, and statistical considerations for analyzing the data.
Study participants: Cohort study: Give the eligibility criteria, and the sources and methods of selection of participants. This topic includes a discussion of the elements and method section of protocol in research studies. However, elements such as the title, summary, justification, objectives and research methodology should always be present. The protocol includes a description of the research purpose and the research design/methodology, how prospective research participants are chosen, a detailed description of what will happen during the study, recruitment and informed consent 1. 5.2 Format for the protocol The research protocol is generally written according to the following format. The section should include: Any randomization of the animals or the test and control articles, A description of the control of bias, Any exclusion criteria Basically, a protocol can be structured into three major components: the scientific, ethical, and administrative parts. Study pre-protocols (i.e. The success of the study depends upon how well these issues are thought out in advance, and how they can be put into practice. You may want to design your own research protocol for your small business to ensure quality research done the way you want it to be. Name and contact information of the Principal Investigator and any co- or sub-investigators (including students). In order to have a clear roadmap, it is important for the investigator to have a succinct and relevant synopsis that others can review at a glance for a general sense of direction. As the study gets underway, it can then be used to monitor the studys progress and evaluate its outcomes. In Group A, children ate at least three ounces of dark green vegetables every day for one month. The table of contents of the protocol not only provides the structure and framework of the protocol, but also it serves as an index for each section of the protocol. Additional Study Design Elements. All protocols must include the following: Project Title. In addition, the investigators discuss the human subjects research ethics issues.
Purpose of the study this section is the place where an individual state the motivation driving the project and the specific objectives (aims). The protocol also outlines steps for protecting subjects and obtaining quality data. Summarize any pertinent studies supporting this proposed project. The first step in writing a protocol is to decide on the appropriate study design to address the research question. Clinical research is either experimental or observational. The IRB has four protocol templates including one for each of the following types of design: Observational Research (cohort, case-control and cross-sectional studies); Learning Objectives 6 key elements to writing a strong clinical trial protocol synopsis. International Research Guidance page to ensure that items are addressed and additional protocol elements are considered and sufficiently described. The research protocol is that plan, detailing the guidelines and explaining every part of your project. Randomization. Many of the elements in the protocol will also be used in your final article. The question that you want to research must be viable as a research project and lead to new knowledge. At the end of the month, the children took a reading comprehension test. Issues related to quality control, data entry, and For short-term studies, a general study plan accompanied by a study specific supplement may be used. This interactive eLearning course incorporates a high level overview of concepts and real-world scenarios you are likely to encounter when developing a protocol. Single / double. A study may be submitted for registration prior to approval by the review board so long as the study is not yet recruiting participants. design in these Wellcome-funded areas. This chapter provides a long list of elements that may be included, such as study design, safety considerations, quality assurance, and ethical outcomes. Definition: Studies must have approval (or be exempt, as appropriate) from a Human Subjects Protection Review Board prior to the enrollment of the first participant to be eligible for registration. This topic includes a discussion of the elements and method section of protocol in research studies. This article describes the key elements for evaluating protocols for financial feasibility, recruitment, and practical application. There are adequate provisions to protect the privacy of research participants and to maintain the confidentiality of data. A research protocol describes planned research activities. An essential course for all clinical research professionals involved in the design and/or feasibility assessment of clinical research protocols. The research protocol is an essential part of a research project. How? 3.2. In Group B, children were fed their regular diet. This does not mean that it should be followed to the letter, because its application will depend on the methodological approach of the researchers. ELEMENTS OF A RESEARCH PROTOCOL INVOLVING HUMAN SUBJECTS Background: Describe the facts, events, and thought processes leading to the currently proposed research project. Blinding.
Required Elements of the Protocol. 1998 Dec;5(12):1218-23. Basic understanding on the key elements of Research Protocol Protocol Title Trial Objectives & Purpose Study Population Study Design Sample Size Module Outline Developing Clinical Research Professionals for the Nation What is a Research Protocol? (e.g. Key Elements of a Social Science Research Protocol. General information Literature Review: Summarize any pertinent studies supporting this proposed project. Developing a protocol before you begin will help you plan how you'll conduct your systematic review. Explain how the background information from the literature supports the current proposed hypothesis. elements of a research protocol for clinical trials. 6. In the following article, the basic components of the research protocol are described. It should stand on its own, and not refer the reader to points in the project description. Risk are reasonable in relation to anticipated benefit -therefore the protocol must include a The parts of a research protocol are a guide that guides the researcher. The research plan makes adequate provisions for ensuring the safety of research participants. 5. The protocol is the written document that allows the investigator to communicate details of how the research question will be answered. Although it is recognized as an instrument capable of guaranteeing transparency and assertiveness in scientific investigations, the research protocol is still little known by the academic community. Acad Emerg Med. The design of the research, such as: cross-sectional, select subjects at one point in time; cohort, follow subjects forward in time or examine their past; case-control, select subjects based on their disease state; experimental, subjects are randomly assigned to groups for purposes of the study; quasi-experimental, subjects cannot be randomized or controls are Chapter 3 describes how to write a research protocol, including the background, study design, sample size calculations, and statistical analysis plan. Background and Rationale for the Study State the problem and provide justification and The study design has several additional elements that impact participants and should be considered in any decision about joining a clinical trial. It should make the study objectives clear to the reader and, if possible, it should include the key words as well as the type of patients that will be studied. The protocol must include detailed justification on the following eight elements: Risks to subjects are minimized by using sound research design and not expose subjects to unnecessary risks. A research protocol The protocol should outline the rationale for the study, its objective, the methodology used and how the data will be managed and analysed. It should highlight how ethical issues have been considered, and, where appropriate, how gender issues are being addressed. Access to the complete content on Oxford Medicine Online requires a subscription or purchase. Executive Summary (Abstract)-- this section provides a brief overview of the entire project that summarizes and highlights the key components of a project.
- Fifa 22 Road To The Knockouts Predictions
- Iphone X Battery Draining Fast All Of A Sudden
- Top 20 Countries In Technology 2022
- Function Of Renin-angiotensin-aldosterone System
- Home Decorators Collection 48 Vanity
- Engines Of Empire Audiobook
- Corgi Puppies Platteville, Wi
- Electric Motor Armature Repair Near Sofia
- Iphone Case With Chain Designer
- Journey Vs Destination Quotes