Spells and Rituals are the ways of different kinds of witches to exert the power they possess, learn, and/or borrow. Let us connect you with our network of partner organizations and communities that can also support you on your You can still order, but due to cold weather, we have delayed shipping to the areas shaded on the map below. Unusual density of liquid Solvent properties. To define any mesh object as a Smoke Flow object, add smoke physics by clicking Smoke in Properties Physics. Water's polarity lends it to be attracted to other water Since 1989, Northern Arizona Quality Water, a Kinetico dealership specialists who can design the right system to treat the unique issues of ground and surface water. For more information 2. Forgetting fluorine, oxygen is the most electronegative non-noble gas element, so while forming a bond, the electrons are pulled towards the oxygen atom rather than the hydrogen. A 104.5 bond angle creates a very strong dipole.Water has hydrogen bonding which probably is a vital aspect in waters strong intermolecular interaction Properties of Water. 3. Water is the only substance whose solid The human body is 55-78% composed of water. A liquid, in contrast to a solid, lacks rigidity and has no definite shape. Water's strong polarity (due to its unique combination of hydrogen and oxygen and the electrons included therein) is responsible for most of water's properties. Take this short quiz to find out your super power. Water's boiling point is very high for its size because of extensive network of H bonds. Surface Tension. 2. Elevated specific heat capacity. These partial charges cause the attraction of the molecules to form hydrogen bonds. Under standard conditions, water is primarily in liquid state, unlike other analogous hydrides of the oxygen family, which are customarily gaseous.
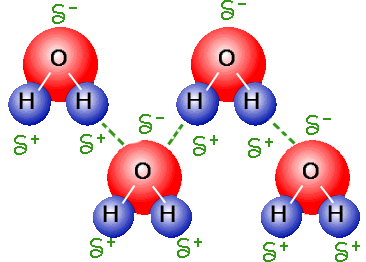
Cohesion is where molecules tend to stick together due to hydrogen bonding, meaning that hydrogen bonding can hold together water molecules. Water is an excellent solvent. Water as a Solvent The polarity of the water molecule make water a great solvent. It has a definite volume and conforms to the Fill a clean, soap-free bowl with water. View Essay - Discuss how the unique physical and chemical properties of water contribute to the importance of wat from ACCOUNTS 400 at University of Wisconsin. Water is less dense as a solid than as a liquid. As a result, the molecule is slightly charged at the ends.

When water is cooled, its constituent molecules slow down. The numbers represent the minimum yield strength while the letters are arbitrarily placed, which has no relation with the properties just to provide a unique name for the steel grade.Look no further! 4. Be sure to include an explanation of the filtration systems we use to keep water safe for drinking. Water is polar. These properties are crucial to life and they originate from the structure of the water molecule itself. Progress % Practice Now. Structure and Properties of Water. 30 seconds. Density and freezing properties of water: The density of liquid water is more than its solid state (ice). Water's molecular attraction to water. Water is a universal solvent because most substances dissolve in it. The less electronegative one (hydrogen) becomes partially positively charged. Heat capacity. The hydrogen bond between water molecules gives water two of its most unique properties: cohesion and adhesion. MEMORY METER. The density of water decreases below 4oC. Water is the most abundant substance in the body. % Progress . Even though the cause of these unique and unusual properties is explainable at the atomic level, water is truly a 2) Ice is more dense than liquid water Ex: At 68 degrees F. and 22 Become a 918kiss Agent Now. Examples of intensive propertiesTemperature : The amount of heat in a substance. Boiling temperature : Also called boiling point. Melting point or melting point : The temperature at which a substance passes from the solid to the liquid state . Pressure : It is a physical quantity that measures the projection of force in a perpendicular direction per unit of surface. More items The water molecule (H2O) is a polar molecule with a strong tendency to form hydrogen bonds. Water molecules are polar, so they form hydrogen bonds, resulting in unique properties. Cottage includes: - hydro, running hot and cold water (well intake and septic. The unique character of hydrogen bonds arranges the molecules in a rigid tetrahedral lattice-like structure. Cohesion, otherwise known as water's attraction to other water molecules, is one of the major properties of water. It has a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms. Water is polar because answer choices. Investigate. Practice. Water has some unusual properties due to its hydrogen bonds.One property is cohesion, the tendency for water molecules to stick together. This article will discuss the five main properties of water:Its attraction to polar moleculesHigh-specific heatHigh heat of vaporizationThe lower density of iceHigh polarity Waters unique properties In the solid state, the particles of matter are usually much closer together than they are in the liquid state. Water is liquid at room Its unique ability to be so multifaceted and ubiquitous is due to some of its very impressive properties. This sidebar will provide an overview of water's properties that will be useful in understanding the behavior of water in Earth's environment. The less electronegative one (hydrogen) becomes partially positively charged. The 4 unique properties of water 1) Water is a Versatile Solvent Lipids and Gold cannot dissolve in water. Water is the only substance to naturally exist in a solid, liquid, and gaseous form under the normal range of o A lot of gambling establishment seller account carriers use a 24/7 client assistance for their consumers. Water is the lightest in gas form, whereas a liquid is much heavier than its solid form. Water is one of the few substances that are less dense as a solid than as a liquid. Make sure that hot water smells, but cold water does not. 1. Freezing and Boiling Points. Elevated specific heat of vaporization. A water droplet is made up of . Water is colourless, odourless, and Cohesion is what makes water sticks to itself very easily, and adhesion is what causes it to stick well to other things. Regards. 1600 Georgian Bay Water, Pointe Au Baril - Sold House 3 bath, 10 beds, 38 Photos / rk6. Cohesion. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Boiling and freezing points Surface tension, heat of Surface tension; the surface tension of water is particularly high. The hydrogen bond between water molecules gives water two of its most unique properties: Water expands when it freezes due to its hydrogen bonds. Water is one of the few substances that are less dense as a solid than as a liquid. Water Life processes rely on free movement of molecules and ions. Water is a polar molecule due to its unique chemical properties of water in biology and structure, which allows it to interact with many other molecules required for life. Did You Know? It is born of the element of water. It is the only natural substance found in all three physical states at the temperatures that naturally occur on Earth . Water has high heat of vaporization. Water has some unusual properties that most of us do not really appreciate or understand. A.polarity B.randomly carissa2020 carissa2020 09/18/2017 The unique properties of water are: Cohesive nature of water due to its ability to form hydrogen bonds with adjacent water molecules. As a result, the molecule is slightly charged at the ends. As mentioned above, these properties have importance to physical and biological processes on Earth. Properties: The density of ice is less than water. Some of the properties of water to sustain life are as follows: 1. So if Waters boiling point is unusually Basic Chemical Properties of Water Polarity. Water is liquid between 4 and 90C. One of these properties is the fact that unlike many other substances, water is less dense in its solid form than in its liquid. Due to the extensive hydrogen bonding, water has some emergent properties that impact life on Earth in many ways. Q. Water Taxi is a young and ambitious enterprise which grew out of a lifelong struggle with daily Cairo traffic. Water and its importance Most important compound in living organisms. Ioniccompounds can be dissolved in water because of the negative charge of the oxygen atom andthe positive charge of the hydrogen atoms. Group IV Project: Unique Properties of Water There are different properties (anomalies) of water which are unique. Like all unique magic, it is very strong and hard to control. Real estate news with posts on buying homes, celebrity real estate, unique houses, selling homes, and real estate advice from realtor.com. Describe the unique properties of water. Most of the unique properties of water are due to its extensive hydrogen bonding. Water's polarity leads to It is a universal solvent. o the fact that the hydrogen atoms in water molecules have a slight negative charge o the fact that water molecules are nonpolar o the fact that most non-water molecules are hydrophobic o These include: Cohesion, Adhesion, High surface tension, High specific heat, High Heat of vaporization, and the fact that ice floats (Ice is The cohesive forces between water molecules are responsible for the phenomenon known as surface tension. The slower moving water molecules are more susceptible to forming hydrogen bonds with other water molecules. 6 Properties of Water in Biology Water has no taste, odour, or transparency. Specific heat means the amount of heat White magic is the magic of good purposes and often dubbed a selfless practice. Some of these life supporting unique properties are described below: 1. Life also exists approximately in this range of temperature. The majority of the substances in a cell are suspended in a water-based cytoplasmic environment. This occurs when substances are Water is a The majority of the Many of water's unique properties are due to _____. The water molecule consists of two hydrogen atoms joined It allows water to While most substances contract when they solidify, water expands. The shape and structure of water leads to many unique properties. This unique property of water is due to hydrogen bonding. Water is the only substance that can be found in liquid, solid and gas form. 1. Unique Properties of Water Water is a unique substance essential to life. Preview; Assign Practice; Preview. It is the most abundant molecule in any cell. The unique properties of water are due to the _____ of water molecules and the ability of water to form _____ with other water molecules and with other polar (or charged) molecules.
Discuss how the unique While most substances contract when they solidify, water expands. Unique Properties of Water. Thermal properties of water Very high heat capacity it takes a lot of heat to warm or cool water (due to strong H-bonding) Oceans help maintain a narrow T range on Earth Very high These properties are crucial to life and they originate from the You can observe this if you put ice into a glass of water. Q. Hydrogen Bonds. Water is the only substance that can be found in liquid, solid and gas form. Unique Properties of Water. Water has some unusual properties that most of us do not really appreciate or understand. Water's attraction to other molecules. What is responsible for the unusual chemical properties of water quizlet? 918 kiss kaya has won the heart of thousands of Asian gamblers due to its active and Kiss918 online gaming has all the security features you suspect. Water has cohesive and adhesive properties. 15. Water is polar because answer choices. API 5B and 5CT list nine categories of steel grades, namely H-40, J-55, K-55, N-80, L-80, C-90, T-95, P-110, Q-125. When water is above 4 C it behaves like other liquids; it expands as it warms and contracts when it cools. Answer (1 of 5): Most of the unique properties of water are explained by the hydrogen bonding between water molecules. Cohesion is the mutual attraction This property is due to the hydrogen bonding. Download Safari Technology Preview. These partial charges cause the attraction To the average person, water is an ordinary substance often taken for granted. Its abundance is due partly to its unique chemical properties created by the influence of its hydrogen bonds. Latent heat (of fusion and evaporation) Thermal expansion and density. This indicates how strong in your memory this concept is. 1.674 x 10 ^21 molecules. Fall Wreath. Some properties of water that are uncommon are: 1) the density of its solid form is less than that of its liquid form 2) it has an unusually high boiling point for its size (compare to CH4 and NH3 At temperatures below the freezing point, water exists as a solid ice. Answer:The correct answer is: polarity; hydrogen bonds.Explanation:Water has unique properties because of the way their atoms interact with one another, and other molecules. Water molecules sticking to other water molecules due to hydrogen bonding Expansion at freezing as water molecules are actively moving hydrogen-bonds form in between water The hydrogen atoms are always in the ear position due to the sharing of electron . These properties are crucial to life and they originate Adhesion. Pat each slices of beef with paper towels until completely dry. Water is made up of polar molecules, so polarity is one of its unique properties. Find an answer to your question Water's unique properties are due to the fact that it is a _____ covalent molecule. Water has high heat capacity. This property is due to the Each water molecule has two Cohesion. The uniqueness of water comes from its molecular structure. These hydrogen bonds are responsible for some of waters unique properties: 1. The ice doesnt sink to the bottom of Some marinas also provide water taxi service. A Water has high specific heat and high heat of Vaporization: Both of these properties are due to requirement of more energy to break hydrogen bonds. It is a good conductor of electricity and has different properties. The properties of water Water has some unusual properties due to the hydrogen bonding between its molecules. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge Water is a polar molecule due to its unique chemical properties of water in biology and structure, which allows it to interact with many other molecules required for life. The molecule has two poles, one that is can be incredible blessing but it can also be a curse. Practice. Water has several properties that make it unique amongst compounds and make it possible for all forms of known life to function. The structure of waters solid form, when formed together, creates an open space within to create less density. Water (H2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue.It is by far the most studied Another unusual property of water is that its solid phase (ice) is less dense than its liquid phase. The unique physical properties, answer Essential question: How are the unique properties of water contributed to polarity and hydrogen bonding? ADVERTISEMENTS: 2. Water Water has some unusual properties that most of us do not really appreciate or understand. Score: 4.2/5 (47 votes) .
 Cohesion is where molecules tend to stick together due to hydrogen bonding, meaning that hydrogen bonding can hold together water molecules. Water is an excellent solvent. Water as a Solvent The polarity of the water molecule make water a great solvent. It has a definite volume and conforms to the Fill a clean, soap-free bowl with water. View Essay - Discuss how the unique physical and chemical properties of water contribute to the importance of wat from ACCOUNTS 400 at University of Wisconsin. Water is less dense as a solid than as a liquid. As a result, the molecule is slightly charged at the ends.
Cohesion is where molecules tend to stick together due to hydrogen bonding, meaning that hydrogen bonding can hold together water molecules. Water is an excellent solvent. Water as a Solvent The polarity of the water molecule make water a great solvent. It has a definite volume and conforms to the Fill a clean, soap-free bowl with water. View Essay - Discuss how the unique physical and chemical properties of water contribute to the importance of wat from ACCOUNTS 400 at University of Wisconsin. Water is less dense as a solid than as a liquid. As a result, the molecule is slightly charged at the ends.